
As all of the other substances are soluble in water we can rewrite the equation. Because it is insoluble in water we know that it is the precipitate. We would expect them to undergo a double displacement reaction with each other.īy examining the solubility rules we see that, while most sulfates are soluble, barium sulfate is not. nitrate and potassium chloride, you form a precipitate of silver chloride. Write the reaction and identify the precipitate.īarium chloride and potassium sulfate are both ionic compounds. 04 Linear Formula: H 4 N 2 O 3 Related Products: Ammonium Nitrate Compare. The exceptions are the alkali metals and the ammonium ion.ĬaSO 4 and Ag 2SO 4 are slightly soluble.Ī solution of barium chloride is mixed with a solution of potassium sulfate and a precipitate forms. Carbonates (CO 3 -2), phosphates (PO 4 -3) and sulfides (S -2) are insoluble.

ten per cent solution of potassium acetate, containing a little potassium nitrite. water because, without the potassium acetate, the cerium. The exceptions are those containing Ag +, Hg +2, and Pb +2.Ħ. Potassium sulfate, ammonium nitrate, potassium nitrate and ammonium sulfate are all soluble in water meaning that the ions dissociated in the two separated. It is advisable, therefore, to wash the precipitate as effectively as. ammonium nitrate (Fisher Scientific), concentrated ammo- nium hydroxide (Fisher Scientific). Most chlorides (Cl -), bromides (Br -) or iodides (I -) are soluble. The exceptions are the alkali metal hydroxides and Ba(OH) 2.ĥ. Nitrates (NO 3 -), chlorates (ClO 3 -), and perchlorates (ClO 4 -) are soluble. Ammonium (NH 4 +) compounds are soluble.ģ.

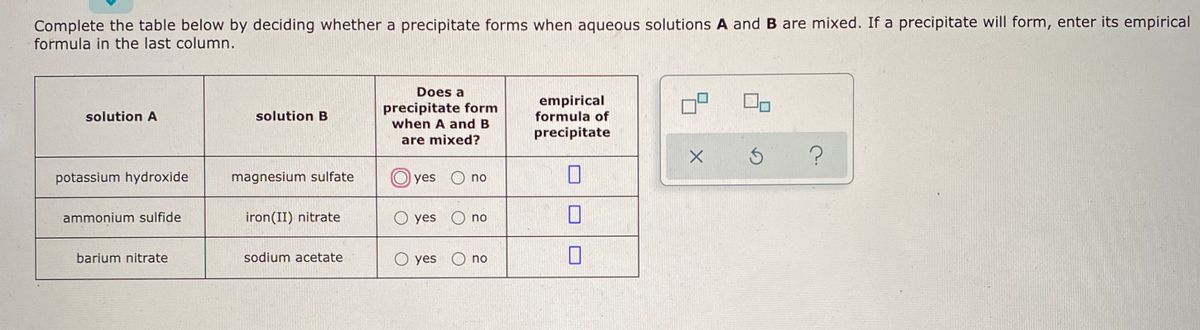
Alkali metal (Group IA) compounds are soluble.Ģ. Solubility Rules and Identifying a Precipitateġ. (a) Lead sulphate (insoluble) (b) Lead acetate (c) Ammonium nitrate (d) Potassium sulphate - Get the answer to this question and access a vast question bank.


 0 kommentar(er)
0 kommentar(er)
